Hyperconjugation involves overlap of
Home » Query » Hyperconjugation involves overlap ofYour Hyperconjugation involves overlap of images are available in this site. Hyperconjugation involves overlap of are a topic that is being searched for and liked by netizens now. You can Get the Hyperconjugation involves overlap of files here. Download all free images.
If you’re searching for hyperconjugation involves overlap of images information related to the hyperconjugation involves overlap of keyword, you have visit the ideal blog. Our site frequently provides you with hints for seeing the highest quality video and picture content, please kindly hunt and find more enlightening video content and images that fit your interests.
Hyperconjugation Involves Overlap Of. The kind of delocalization involving sigma bond orbital is called hyperconjugation. Can you explain this answer. CH or CC with an adjacent unpopulated non-bonding p or antibonding σ or π orbitals to give a pair of extended molecular orbitals. Hyperconjugation involves overlap of the following orbitals.
![]() Organic Chemistry Nature Of Bonding And Stereochemistry Hyperconjugation Part 1 Examrace From examrace.com
Organic Chemistry Nature Of Bonding And Stereochemistry Hyperconjugation Part 1 Examrace From examrace.com
The correct IUPAC name of the compound is a. σ σ b. The number of sigma and pi-bonds in 1-butene-3-yne are. Hyperconjugation involves delocalization of σ σ and π π -bond orbitals ie it undrgoes σ π σ - π conjugation. Hyperconjugation involves overlap which of the following orbitals. Correct Answer - B.
A σ σ b σ p c p p d n n.
Option B is the correct answer. Are solved by group of students and teacher of JEE which is. Click to rate this post. Option B is the correct answer. Hyperconjugation involves overlap which of the following orbitals. AnsweredJun 22 2019by Mihika Sahu692kpoints selectedJun 22 2019by xD4y4.
Source: www1.biologie.uni-hamburg.de
AnsweredJun 22 2019by Mihika Sahu692kpoints selectedJun 22 2019by xD4y4. Through this overlap individual electrons can to an extent help bind together four nuclei. These trends and many others arise from extended orbital overlap. Which type of isomerism is possible in CH 3 CHCHCH 3. Can you explain this answer.
 Source: researchgate.net
Source: researchgate.net
Hyperconjugation involves the overlap of the vacant 2p orbital with a neighboring CH σ-bond orbital Fig. Option B is the correct answer. Can you explain this answer. Check Answer and Solution for ab. Hence from the above discussion we can consider that hyperconjugation involves overlap of sigma -p orbitals ie.
 Source: youtube.com
Source: youtube.com
Which type of isomerism is possible in CH 3 CHCHCH 3. π π Answer. It is also called σπ conjugation or no-bond resonance. Is there an error in this question or solution. The main applications of hyperconjugation is it can be used to rationalize a variety of chemical processes which involves anomeric effect gauche effect beta silicon effect and also tells about the stability of.
 Source: toppr.com
Source: toppr.com
Mesometic effect involves delocalisation of a pi-electrons b sigma-electrons c protons d None of these. Hyperconjugation involves overlap which of the following orbitals. Which type of isomerism is possible in CH 3 CHCHCH 3. Are solved by group of students and teacher of JEE which is. The Questions and Answers of Hyperconjugation involves overlap of the following orbitalsaσ-σbσ - πcp- pdπ-πCorrect answer is option B.
Source: quora.com
Basic Principles of Organic Chemistry - Exercises Page 232 Q 15. Hyperconjugation involves overlap of σ - p orbitals. Option B is the correct answer. Is there an error in this question or solution. Hyperconjugation involves overlap of the following orbitals a σ-σ b σ- π c p- p d π-π.
 Source: toppr.com
Source: toppr.com
The number of sigma and pi-bonds in 1-butene-3-yne are. Delocalization of this kind involving a sigma bond orbital we recognize as hyperconjugation. Hyperconjugation involves overlap which of the following orbitals. Theoretical Basis of Organic Reactions. Please log inor registerto add a comment.
 Source: youtube.com
Source: youtube.com
Option B is the correct answer. Hyperconjugation involves overlap of σ - p orbitals. Hyperconjugation involves overlap of the following orbitals. CH or CC with an adjacent unpopulated non-bonding p or antibonding σ or π orbitals to give a pair of extended molecular orbitals. A σ σ b σ p c p p d n n.
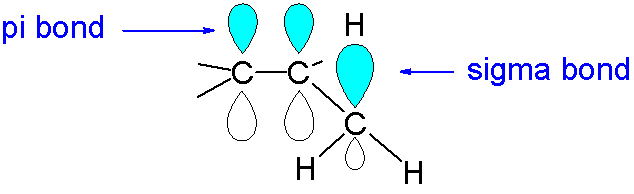 Source: en-academic.com
Source: en-academic.com
In organic chemistry hyperconjugation or σ-conjugation refers to the delocalization of electrons with the participation of bonds of primarily σ-character. π π Answer. Basic Principles of Organic Chemistry - Exercises Page 232 Q 15. Hyperconjugation involves overlap of the following orbitals a σ-σ b σ- π c p- p d π-π. Can you explain this answer.
 Source: toppr.com
Source: toppr.com
Option B is the correct answer. The best way to gain an appreciation of the structural requirements for hyperconjugation is to inspect. The Questions and Answers of Hyperconjugation involves overlap of the following orbitalsaσ-σbσ - πcp- pdπ-πCorrect answer is option B. Hyperconjugation involves overlap of. Hence from the above discussion we can consider that hyperconjugation involves overlap of sigma -p orbitals ie.
 Source: youtube.com
Source: youtube.com
Hyperconjugation Involves Overlap of the Following Orbitals The hyperconjugation involves the overlap of the σp orbital. It is also called σπ conjugation or no-bond resonance. Option B is the correct answer. π π Answer. It is also called σπ conjugation or no-bond resonance.
![]() Source: examrace.com
Source: examrace.com
Hyperconjugation involves overlap of the following orbitals. The correct IUPAC name of the compound is a. It is also called σπ conjugation or no-bond resonance. Basic Principles of Organic Chemistry - Exercises Page 232 Q 15. Hence from the above discussion we can consider that hyperconjugation involves overlap of sigma -p orbitals ie.
 Source: pinterest.com
Source: pinterest.com
Correct Answer - B. Hyperconjugation involves overlap of the following orbitals A σ - σ - askIITians. Hyperconjugation involves overlap of the following orbitals a σ-σ b σ- π c p- p d π-π. The main applications of hyperconjugation is it can be used to rationalize a variety of chemical processes which involves anomeric effect gauche effect beta silicon effect and also tells about the stability of. Hence from the above discussion we can consider that hyperconjugation involves overlap of sigma -p orbitals ie.
Source: quora.com
These trends and many others arise from extended orbital overlap. S-p Conjugation Carbocation Stabilities. Hence from the above discussion we can consider that hyperconjugation involves overlap of sigma -p orbitals ie. Is there an error in this question or solution. The Questions and Answers of Hyperconjugation involves overlap of the following orbitalsaσ-σbσ - πcp- pdπ-πCorrect answer is option B.
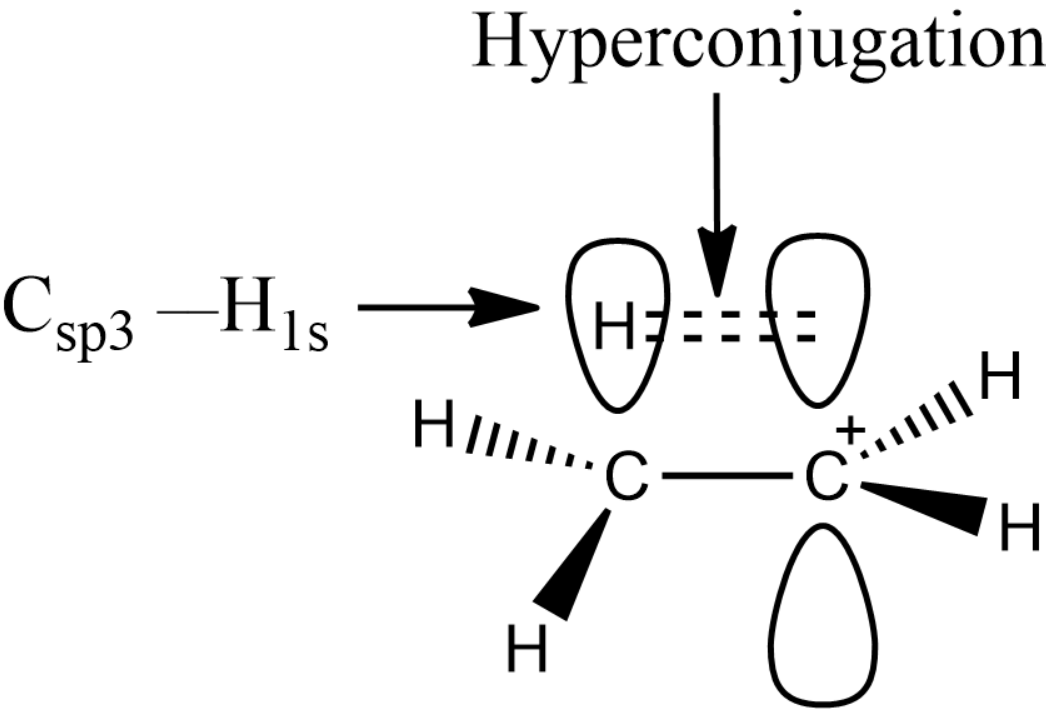 Source: vedantu.com
Source: vedantu.com
Hence from the above discussion we can consider that hyperconjugation involves overlap of sigma -p orbitals ie. Which type of isomerism is possible in CH 3 CHCHCH 3. It is also called σπ conjugation or no-bond resonance. The best way to gain an appreciation of the structural requirements for hyperconjugation is to inspect. The number of sigma and pi-bonds in 1-butene-3-yne are.
 Source: sciencedirect.com
Source: sciencedirect.com
Delocalization of this kind involving a sigma bond orbital we recognize as hyperconjugation. Hyperconjugation involves overlap which of the following orbitals. Click to rate this post. Hyperconjugation involves overlap of the following orbitals. Hyperconjugation involves overlap of σ - p orbitals.
 Source: wikiwand.com
Source: wikiwand.com
Hyperconjugation Involves Overlap of the Following Orbitals The hyperconjugation involves the overlap of the σp orbital. The number of sigma and pi-bonds in 1-butene-3-yne are. P p d. Hyperconjugation involves overlap of the following orbitals A σ - σ - askIITians. Can you explain this answer.
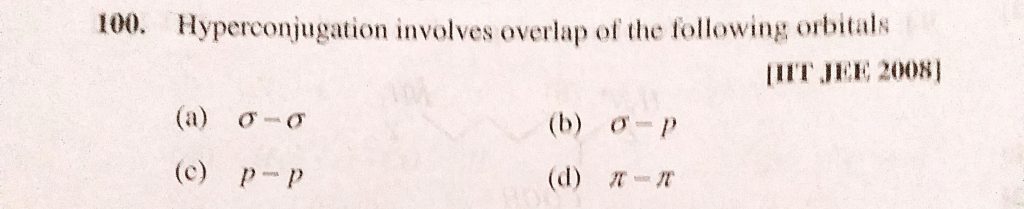 Source: sahay.guru
Source: sahay.guru
Are solved by group of students and teacher of JEE which is. Is there an error in this question or solution. Hyperconjugation involves overlap of the following orbitals A σ- σ B σ - p C p- p D π - π. In organic chemistry hyperconjugation or σ-conjugation refers to the delocalization of electrons with the participation of bonds of primarily σ-character. Are solved by group of students and teacher of JEE which is also the largest student community of JEE.
 Source: pinterest.com
Source: pinterest.com
Correct Answer - B. The correct IUPAC name of the compound is a. It is also called σπ conjugation or no-bond resonance. Hyperconjugation involves overlap of the following orbitals. Delocalization of this kind involving a sigma bond orbital we recognize as hyperconjugation.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title hyperconjugation involves overlap of by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.
Category
Related By Category
- Apex legends how to unlock characters
- Organismic valuing process
- Flag hey dudes womens
- What does flying the american flag upside down mean
- Queen of trash elmo
- American flag with blue stripe meaning
- Hey dude womens wendy patriotic stars and stripes
- Roland kubler now
- 365 days adrianna bartkowska
- Tyson spicy chicken patties recall